Prevalence of and risk factors for paratuberculosis in purebred beef cattle
Objective—To estimate the prevalence of paratuberculosis in purebred beef cattle in Texas and identify risk factors for seropositivity.
Design—Epidemiologic survey.
Animals—4,579 purebred cattle from 115 beef ranches in Texas.
Procedure—Blood was collected, and serum was analyzed for antibodies with a commercial ELISA. Fecal samples were collected and frozen at −80°C until results of the ELISA were obtained, and feces from seropositive cattle were submitted for mycobacterial culture. Herd owners completed a survey form on management factors.
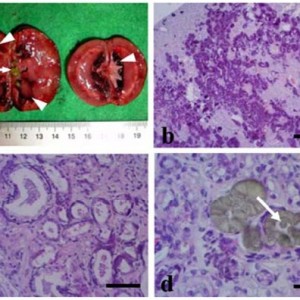
Results—Results of the ELISA were positive for 137 of the 4,579 (3.0%) cattle, and 50 of the 115 (43.8%) herds had at least 1 seropositive animal. Results of mycobacterial culture were positive for 10 of the 137 (7.3%) seropositive cattle, and 9 of the 50 (18%) seropositive herds had at least 1 animal for which results of mycobacterial culture were positive. Risk factors for seropositivity included water source, use of dairy-type nurse cows, previous clinical signs of paratuberculosis, species of cattle (Bos taurus vs Bos indicus), and location.
Conclusions and Clinical Relevance—Results suggested that seroprevalence of paratuberculosis among purebred beef cattle in Texas may be greater than seroprevalence among beef cattle in the United States as a whole; however, this difference could be attributable to breed or regional differences in infection rates or interference by cross-reacting organisms. Veterinarians should be aware of risk factors for paratuberculosis as well as the possibility that unexpected serologic results may be found in some herds. (J Am Vet Med Assoc 2005;226:773–778)
Authors: Dr. Allen J. Roussel, Dr. Melissa C. Libal, Robert L. Whitlock, Thomas B. Hairgrove, Kerry S. Barling, James A. Thompson
Source: https://avmajournals.avma.org/











List
Add
Please enter a comment