Efficacy of L-Asparaginase administration for feline gastrointestinal lymphoma
L-Asparaginase (L-Asp) is often used to induce remission in feline large-cell gastrointestinal lymphoma (LCGIL). However, no study has evaluated the efficacy and adverse events following the initial use of this drug as a first-line treatment in feline LCGIL.
This authors retrospectively reviewed medical records of cats with LCGIL treated with L-Asp to induce remission.
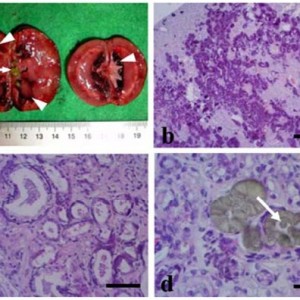
The study included 43 cats. The response rate (RR) after the first administration of L-Asp was 37.2% (Complete remission: 7.0 %, partial remission: 30.2%). RR was significantly higher in cases with primary gastric lesions (64.3%) than in those with primary intestinal lesions (24.1%) (P=0.018), and it was also higher in cases without anemia (57.1%) than those with anemia (15.0%) (P=0.009). The most common adverse event was hyperammonemia, which occurred in 10 of 12 cases where we could compare plasma ammonia concentrations before and after the first dose of L-Asp. Plasma phosphate concentrations were also significantly increased (P<0.001) within 24 hr after the first dose. Decreased appetite, vomiting, and diarrhea were also observed in five, three, and seven cases, respectively, and Grade 3 or higher gastrointestinal signs were observed as adverse events in three cases. The median overall survival of all cats was 150 days (range, 5-1,065 days), and the median progression-free survival was 104 days (range, 2-978 days).
In conclusion, L-Asp was effective to induce remission, and severe adverse events were uncommon in feline LCGIL.
“Efficacy and adverse events of L-Asparaginase administration as a first-line treatment for feline large-cell gastrointestinal lymphoma”. Haruka Inazumi, et al. J Vet Med Sci. 2024 Jun 3.
Source: https://www.jstage.jst.go.jp/article/jvms/advpub/0/advpub_23-0453/_article













List
Add
Please enter a comment