USDA grants conditional license for Elanco's canine parvovirus monoclonal antibody
Elanco Animal Health Incorporated announced on May 3 that the U.S. Department of Agriculture (USDA) has provided a conditional license for the first canine parvovirus monoclonal antibody. This is the first and only approved therapeutic solution proven to treat canine parvovirus — one of the most contagious and deadly viruses a dog can contract with a 91% mortality rate if not treated with supportive care.
The treatment is the first monoclonal antibody for Elanco, according to the news release.
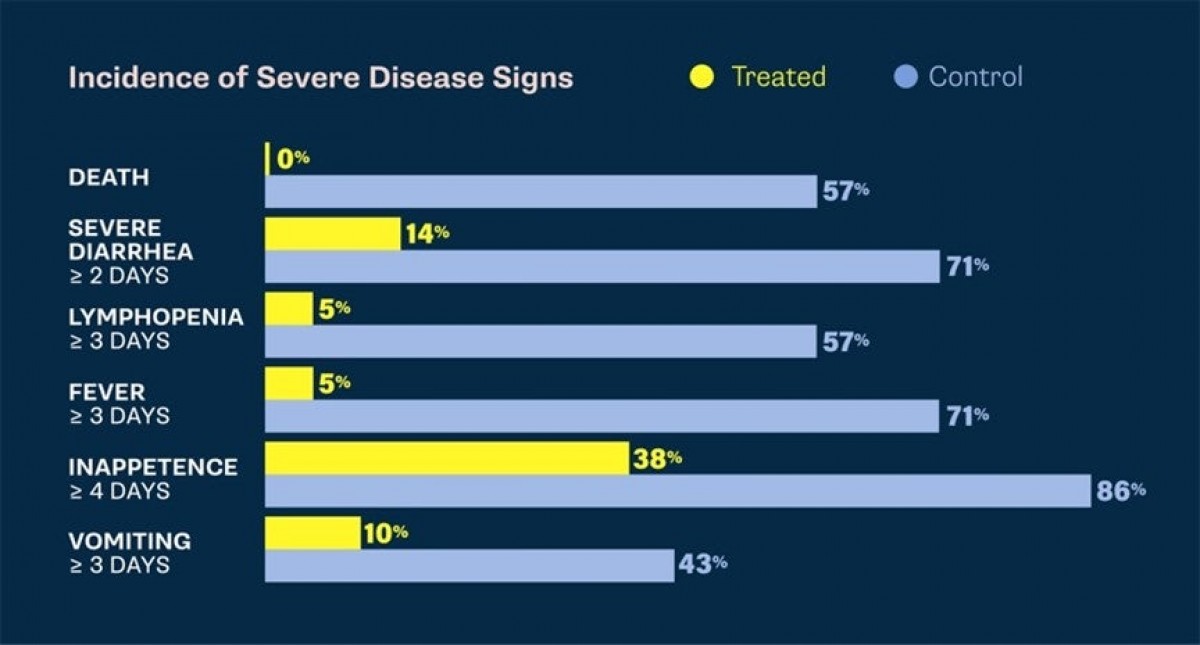
With an estimated 330,000 cases of canine parvovirus in the U.S. annually, clinical trials demonstrate that the canine parvovirus monoclonal antibody single, intravenous dose delivers targeted efficacy in treating this deadly disease. The treatment can be administered to dogs eight weeks of age or older with canine parvovirus. The treatment may provide a less intensive and more effective solution than supportive care alone by targeting the virus with single dose efficacy and a strong safety profile in healthy dogs.
In the news release, Jeff Simmons, president and CEO of Elanco said the treatment will help save puppies lives, while alleviating the emotional and financial burden of both pet owners and veterinary care teams.
The treatment reinforces Elanco’s commitment to pioneer new tools in spaces where there aren’t existing options, he said.
"We’re committed to helping the world’s pets live longer, healthier lives because we believe making life better for our pets makes life better.”
The canine parvovirus monoclonal antibody is expected to begin shipping in the coming weeks, pending individual state approvals. Elanco will also continue to provide canine parvovirus education and resources to veterinarians, shelter owners and pet parents.
Dr. Kristin Zersen, DVM, assistant professor of small animal emergency and critical care at Colorado State University's James L. Voss Veterinary Teaching Hospital, said the announcement is a game changer for pet owners and veterinarians.
“Parvovirus is an unpredictable disease that causes significant strain on pet owners and is labor intensive and stressful for shelters and veterinary clinic staff," she said in the news release. "It’s lifechanging and industry-defining to be able to offer a proven solution to canine parvovirus that limits the need for hospitalization, reducing the impact this disease has on hundreds of thousands of dogs each year.”
The conditional license approval — granted by the USDA to effectively and safely meet an emergency situation, limited market or special circumstance — bridges Elanco’s expertise in both therapeutics and vaccines. In the treatment efficacy study, the monoclonal antibody was proven effective in decreasing mortality associated with parvovirus infection. Treated dogs also had significantly faster times to resolution of the most-adverse effects of parvovirus including vomiting4, meaning that they feel better faster and get home sooner.
Prior to Elanco creating the canine parvovirus monoclonal antibody, the only treatment for the highly contagious canine parvovirus was supportive therapy, which can consist of 24/7 care, multi-day hospitalization and emotional stress for the staff and pet owner with no guaranteed outcome and potentially high pet owner costs.
In conjunction with its portfolio of innovative solutions, this announcement establishes Elanco’s presence in the monoclonal antibody space and reinforces its commitment to helping pets live longer, healthier lives through its portfolio of innovative solutions.
For more information, visit FightParvo.com












List
Add
Please enter a comment