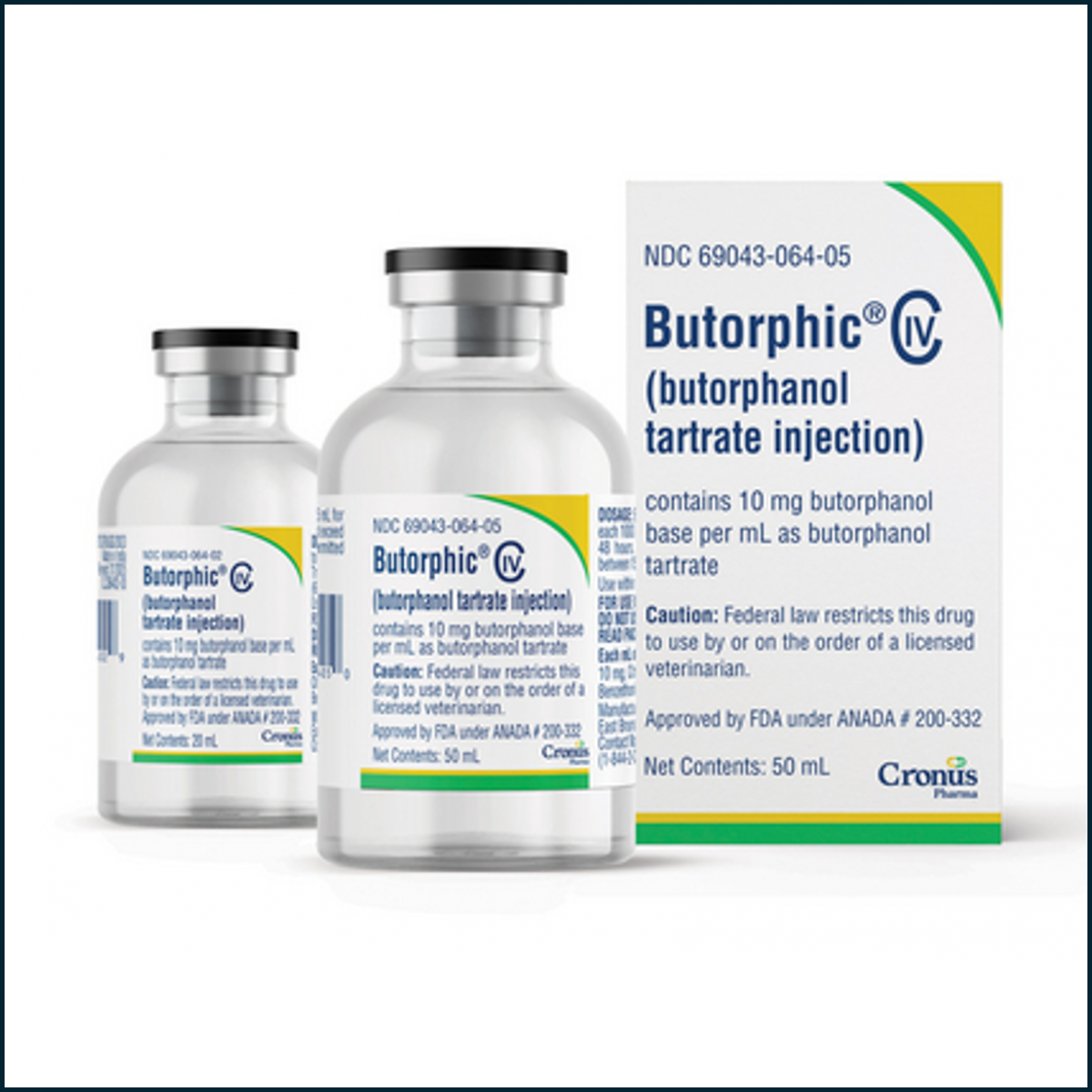
Cronus Pharma Launches Butorphic® (Butorphanol Tartrate) Sterile Injectable Solution
Cronus Pharma LLC, a fully integrated research, development, manufacturing, sales and marketing animal health pharmaceutical company, announces the launch of Butorphic® (Butorphanol Tartrate) Sterile Injectable Solution for immediate availability in the U.S. animal health market through national and regional distributors.
"Butorphic is a vital analgesic for managing equine colic pain and we are excited to grow our equine portfolio. Importantly, Butorphic marks our fifth product launch this year, expanding our portfolio to twenty-five products with several more planned for 2025 and beyond," said Vimal Kavuru, Chairman & CEO.
This launch enhances Cronus Pharma's robust sedative and anesthesia product portfolio which includes AnaSed® Equine (Xylazine) Injection, Cropamezole™ (Atipamezole) Injection, DexmedVet™ (Dexmedetomidine) Injection, DetomiSed™ (Detomidine) Injection, and Ketamine Injection.
"Butorphic's launch reinforces our position among the animal health leaders in U.S. FDA ANADA approvals over the past several years, which is a testament to our team’s dedication to delivering high-quality, cost-effective pharmaceuticals to veterinarians and pet owners," said Edward Neugeboren, Chief Strategy Officer.
Butorphic is indicated for the relief of pain associated with colic and postpartum pain in adult horses and yearlings. Clinical studies demonstrate that butorphanol tartrate effectively alleviates abdominal pain caused by torsion, impaction, intussusception, spasmodic and tympanic colic, and postpartum pain. Approved by the FDA under ANADA #200-332, Butorphic provides the same efficacy and safety, and is a generic equivalent to the reference product, Torbugesic®. Available in 10 mg/mL concentrations in 20 mL and 50 mL multi-dose vials, our unique 20 mL vial offers veterinarians more flexible dosing options.
About Cronus Pharma
Cronus is a fully integrated research, development, manufacturing, sales and marketing animal health pharmaceutical company headquartered in East Brunswick, New Jersey, dedicated to providing high-quality and cost-effective pharmaceuticals serving the companion, equine and production animal health markets. Through our in-house R&D and acquisitions, Cronus has an extensive product portfolio of seventy-seven US FDA approved products as well as numerous products pending approval and a broad development pipeline. Cronus is proud to be among the leaders in U.S FDA ANADA approvals in the animal health industry over the past several years. Our recently built state of the art manufacturing facility located in Hyderabad, India has broad capabilities including solid oral dose, sterile injectables and cephalosporins. Our products are available to veterinarians and pet owners nationwide through national and regional distributors. Our passionate team is committed to growing our business and being among the leading animal health pharmaceutical companies.
Source: www.cronuspharmausa.com












List
Add
Please enter a comment