A vaccine to control the fertility of urban and peri-urban wild boars
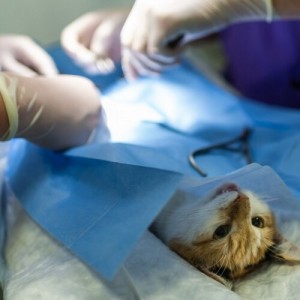
A team of researchers from the Autonomous University of Barcelona (UAB) has released the pilot project results to control the fertility of free-living wild boars in urban and peri-urban areas through immunocontraception treatment. Of a total of 219 project animals, 192 specimens have been vaccinated, of which 56 individuals have been recovered a total of 154 times throughout the study. Immunocontraception with a vaccine was effective in all females and especially among young animals, in which reproductive inhibition could be permanent. In young males, it was also more effective. The project begins a new phase next year to assess whether sterility is reversible or permanent in young specimens before they enter puberty and to study how it can affect their growth and behavior, as reported by the UAB.
The study has been carried out since the end of 2017 in the areas of four municipalities, Terrassa, Matadepera, and Vacarisses -with limits with the Parc de Sant Llorenç del Munt and the Serra de l'Obac- and Sant Cugat del Vallès, -with Collserola Park.
The project has had the support of the Barcelona Provincial Council, the main promoting institution, and has been coordinated by the UAB's Infertility Research Group (GRI-BCN), led by Manel López Béjar from the Department of Animal Health and Anatomy. Members of the National Wildlife Research Center (NWRC) of the USA, the Animal and Plant Health Agency (APHA) of Great Britain, and The Botstiber Institute for Wildlife Fertility Control have also participated.
The project has evaluated the effects of the Gonacon vaccine in wild boars and the transience of these effects. The mechanism of action of the vaccine is the creation of antibodies against gonadotropin-releasing hormone (GnRH), hormones that favor reproductive function in mammals.
The exact duration of the project, which has been affected by the COVID-19 pandemic and aspects related to the importation of the vaccine, has been three years. A total of 219 captured animals have been part of the study. Of the 192 vaccinated animals, 154 recaptures have been carried out throughout the project, which has made it possible to monitor 56 vaccinated animals (34 females and 22 males), plus 18 control animals and 30 individuals exclusively for health studies. The percentage of recaptured animals was 29.2%.
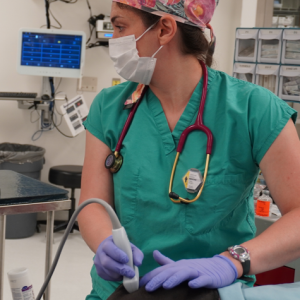
Through the appearance of the external genitalia and the mammary gland, the determination of the hormones related to reproduction, the observation of the tissues of the gonads when it has been possible and the titers of antibodies, it has been possible to determine the effectiveness of the application of the vaccine.
Effects of treatment
Manel López Béjar explained that the pilot project has made it possible to confirm the efficacy of the Gonacon vaccine in inhibiting the reproduction of wild boars. "As for females, the treatment has been evaluated from 4 months to 3 years after the application of the vaccine, depending on the time of recapture. Immunocontraception was effective in all treated females that could be recaptured. Animals that are already adults seem to require annual or bi-annual revaccination to continue to be effective. And a very important finding, from the point of view of efficiency in controlling population growth, is that we have verified that the inhibitory effect on reproduction seems to be permanent when the treated animal is a young animal, around puberty, between 4 and 6 months of age", he stated. "This would lead to less aggressiveness and less occupation of spaces and mobility by contraceptive animals. In fact, in a survey carried out in one of the municipalities collaborating with the project, we verified that the intensification of treatment in urban and peri-urban areas reduces the number of conflicts and the accident rate", he pointed out.
"In males, we have been able to verify the efficacy of the treatment from 2 months to 2 years in at least a dozen specimens, with greater efficacy also when the vaccinated animal is young. Now it remains to confirm and evaluated whether the use of Gonacon in prepubertal animals, between 3 and 6 months of age, leads to a definitive inhibition of reproduction due to lack of development of the reproductive organs, as well as whether this effect leads to changes in behavior and growth in treated individuals", added the researcher. Until this project, immunocontraception has been used exclusively to generate transient infertility in adult animals.
The results of the project will be presented at the next congresses of the International Conference on Wildlife Fertility Control, from May 23 to 25, 2022, in Colorado Springs, USA, and of the Spanish Association of Animal Reproduction (AERA), in León from October 20 to 22, as well as at the 13th International Symposium on Wild Boar and Other Suids, from September 6 to 9, 2022, in Seva, Barcelona. The researchers are also preparing a publication for a scientific journal.
The second phase of the project
The project will begin a second three-year phase in 2022, in which it will seek to verify the effectiveness of the treatment to inhibit the reproduction of prepubertal wild boars when it is administered to young animals between 4 and 6 months of age and determine if this sterility is reversible or persistent also if this effect involves changes in behavior and growth in treated individuals.
The continuation of the study has the same collaborators, NWRC and APHA, in addition to the Botstiber Institute for Wildlife Fertility Control, an international group that aims to mitigate conflicts with wild boar worldwide through fertility control.
The aforementioned collaborators are in the process of publishing results on the contraceptive effect of the oral vaccine in another mammalian species. "If its efficacy is demonstrated, efforts to extend the use of fertility control as a management tool for urban and peri-urban wild boar would be simplified," explained Manel López Béjar. “We could evaluate its application during this second phase, in 2023, which would be a world-first”, he added.
The administration of this vaccine does not represent any risk in relation to the consumption of wild boar meat. The proposed methodology is similar to the one that has been used in Australia and New Zealand for more than a decade in pigs destined for human consumption.






List
Add
Please enter a comment