Gilvetmab: New Treatment for Canine Melanoma and Mast Cell Tumors
by Jenny Alonge
Gilvetmab became available in October to U.S. board-certified veterinary oncologists, after the drug received conditional license approval from the U.S. Department of Agriculture (USDA) Center for Veterinary Biologics (CVB). This innovative approach is the first and only immune checkpoint inhibitor developed to treat mast cell tumors and melanomas in dogs, and has also shown promise in metastatic and late-stage disease scenarios. Keep reading to learn what you need to know about this new product.
What is gilvetmab?
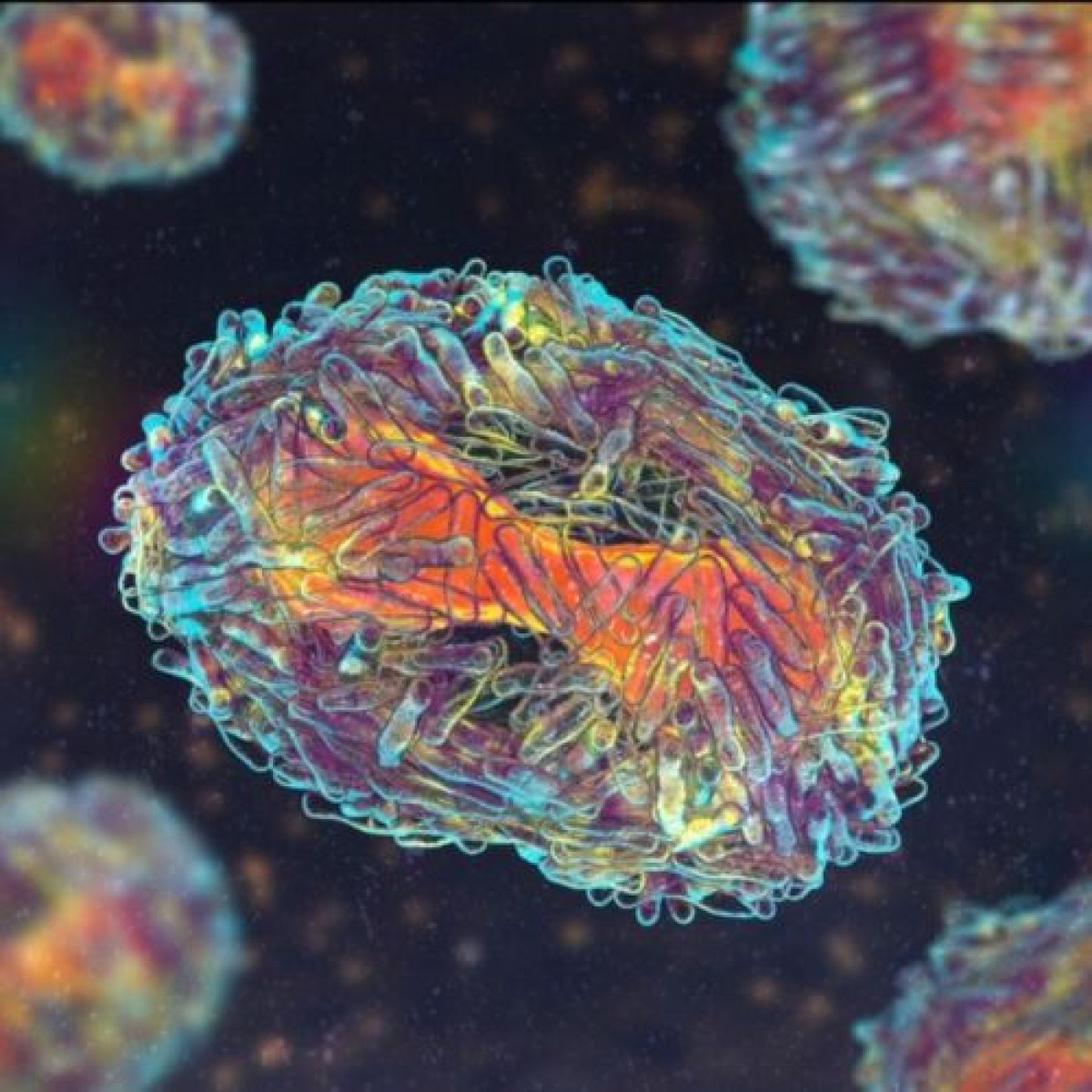
Gilvetmab is a caninized anti-PD-1 monoclonal antibody. The checkpoint inhibitor prevents PD-1 on the T cell from interacting with programmed cell death ligand 1 on the cancer, which allows T cells to regain their ability to kill cancer cells. The product is conditionally approved to treat dogs with stage I, II, or III mast cell tumor, or stage II or III melanoma.
Who has access to gilvetmab?
Currently, gilvetmab is available only to board-certified veterinary oncologists, and treatment requires a veterinary-client-patient relationship (VCPR) with a veterinary oncologist.
How is gilvetmab administered?
Board-certified veterinary oncologists administer gilvetmab as an intravenous (IV) infusion of 4.5 mg/lb or 0.225 mL/lb body weight over a minimum 30 minutes. Diphenhydramine is administered intramuscularly 15 to 30 minutes before the gilvetmab, and the dog is monitored for one hour after infusion for immediate side effects. The product is labeled for use every two weeks for 10 treatments, but the veterinary oncologist can prescribe an extended duration at their discretion.
How effective is gilvetmab?
Clinical efficacy trials demonstrated that gilvetmab exhibited a reasonable expectation of efficacy for solid tumor reduction in dogs with stage I, II, and III mast cell tumors and dogs with stage II and III melanomas. Specific data include:
Mast cell trial — In the initial trial involving 26 client-owned dogs with mast cell tumor, target lesion size decreased or the lesion remained stable in 73%.
Melanoma — In the initial trial involving 25 client-owned dogs with melanoma, target lesion size decreased or the lesion remained stable in 60%.
Is gilvetmab safe?
Safety trials demonstrated that gilvetmab is well-tolerated and safe for dogs. Specific data include:
Beagles — During a safety trial involving 50 healthy beagles, no treatment-related adverse events occurred except in three dogs who displayed mild to moderate allergic reaction signs that resolved quickly with treatment. No other adverse events were noted with repeated infusions.
Client-owned dogs — A field study demonstrated that the 51 client-owned dogs tolerated gilvetmab well. More than 92% of melanoma patient owners and 100% of mast cell tumor patient owners reported that their dog maintained a favorable quality of life during the study.
What are gilvetmab side effects?
Adverse reactions seen during field trials included transient lethargy, inappetence, and gastrointestinal (GI) upset. In laboratory safety studies, some dogs experienced mild facial, eye, or ear swelling and localized erythema.
What dogs are candidates for gilvetmab?
Gilvetmab is indicated for dogs with stage I, II, and III mast cell tumor and stage II and III melanoma. Dogs who need immunosuppressive therapy, such as glucocorticoids or cyclosporine, are not good candidates.
How long does gilvetmab take to work?
Gilvetmab typically is effective in one to two months.
Does gilvetmab cause pseudoprogression?
Immune checkpoint inhibitors cause an influx of immune cells in the tumor, which can get bigger prior to involution. This phenomenon is known as pseudoprogression and was documented in some dogs treated with gilvetmab in the clinical trial.
Can gilvetmab be used with other treatments?
Gilvetmab’s safety and efficacy when combined with other mast cell tumor and melanoma treatments have not been studied.
Can gilvetmab be used to treat other cancers?
Gilvetmab is currently labeled for use only in dogs with melanoma and mast cell tumors, but the Gilvetmab Product Grant Program will provide the product to board-certified veterinary oncologists interested in conducting clinical trials to investigate gilvetmab in other cancers, with adjunct therapies, and as potential biomarkers for successful use.
What should pet owners know about gilvetmab?
Information you can provide your clients about gilvetmab includes:
Gilvetmab is an immunotherapy that uses your dog’s immune system to help detect and fight cancer.
Gilvetmab may take up to one to two months to work, and your dog’s tumor may initially increase in size.
Most dogs in field trials maintained a good quality of life while on gilvetmab, with mild lethargy, inappetence, and GI upset the most common side effects.
Only a board-certified veterinary oncologist can administer gilvetmab treatment, which typically takes two hours per treatment.
The veterinary oncologist determines the treatment frequency.
Monoclonal antibody treatments are an exciting new option for veterinarians, and gilvetmab can hopefully increase longevity and improve quality of life for dogs suffering from melanoma and mast cell tumors.










List
Add
Please enter a comment